The COVID-19 Vaccine: Public Health and ESG Impact
Public Health Outcomes and Other Vaccine-Related ESG Considerations
During the seasonal change from summer to fall, many of us prepare to receive our flu shot—a trip to the pharmacy that is routine in a typical year. As the COVID-19 pandemic rages on, the global search for an effective and safe vaccine races forward, and we continue to grapple with a most atypical and challenging year, vaccines are more top-of-mind for most of us. For centuries, vaccines against a number of human, contagious diseases have successfully and safely provided innumerable public health benefits. The COVID-19 vaccine will be no different once a successful candidate is found and distributed.
THE RACE FOR A VACCINE
For months, public health and safety have been top of mind, with everyone receiving what feels like hourly updates. Under the spotlight of regulatory agencies, media outlets, and even casual conversations, healthcare companies and research institutions the world over are racing to develop a vaccine for SARS-CoV-2 (the disease that causes COVID-19). As the number of global cases rises, and leaders try to prevent an even-higher death toll, the research race at times feels frantic. So far in 2020, the U.S. has spent nearly $6 billion on COVID-19 vaccine research and development;1 in 2019, U.S. spending on all vaccine research and development was approximately $30 billion.2
AN ESG LENS PROVIDES PERSPECTIVE
To the right we highlight our Environmental, Social, and Corporate Governance (ESG) and values-based approach to evaluating corporate social responsibility and constructing responsible investment portfolios. By evaluating material ESG factors for investable companies and for real-world challenges, we better understand how the corporate sector is managing, contributing to, or helping to solve these challenges. An assessment of client values, which sometimes align to general ESG categories, also aids our responsible investing portfolio construction. The healthcare sector faces some ESG risks, ESG opportunities, and values concerns that differentiate it from other industries. A seemingly simple but important act like getting vaccinated has global societal impacts and public health benefits; in this piece we explore many aspects of the vaccine research and distribution processes, with a particular lens of understanding the potentially material ESG factors behind these processes. Shared in the order of material relevance to the vaccines process, we note that many of the ESG questions and ideas presented may not be answered until the COVID-19 pandemic has played out in its entirety.
Environmental, Social, and Corporate Governance (ESG): Commitment to understanding the evolving field of corporate social
responsibility and incorporating material ESG factors in the development of the investment portfolio. Below are examples of potentially relevant factors:
Environmental factors include a company’s exposure to and management of climate change risks, energy use, resource depletion, water, waste, and pollution.
Social factors include company policies related to human rights, workplace safety, product safety, and supply chain management.
Corporate Governance factors include best practices with respect to Board of Director structure and diversity, executive compensation policies, and clearly stated ethics policies.
In addition to ESG risk and opportunity management, responsible investors often incorporate values-based criteria into their investment portfolio. Our approach aims to identify companies whose products and/or activities are consistent with your specific guidelines and to invest in those that support your values.
SOCIAL CONSIDERATIONS
Health & Well-Being
Societal impact perhaps carries the most weight of all ESG factors when discussing vaccines. Keeping global populations healthy is a top priority, especially in a global pandemic. Throughout history, vaccines for various human diseases have proven their societal worth by reducing infections and eradicating extremely harmful diseases. Vaccines provide a valuable public health benefit and a new COVID-19 vaccine would be no different; the companies that produce and distribute vaccines contribute a positive social impact to global health outcomes. One global health outcome, aligned with SDG #3, Good Health and Well-Being, is to ensure healthy lives for people everywhere, which can be achieved through equitable access to quality healthcare and medicines. Some challenges to achieving this access include drug pricing, vulnerable population distribution, and developing markets’ access to medicine. We next explore these social challenges in turn, applying a COVID-19 lens.
Pricing Considerations
Drug pricing debates have occurred for many years, with U.S. pharmaceutical companies taking the brunt of it, due to rising drug costs and the high price of medicines or treatments, which arguably reduce patients’ ability to afford life-saving medical treatments. The main U.S. drug pricing regulation conversations have taken somewhat of a backseat during the COVID-19 pandemic, but there is much global discussion to ensure that companies who develop a successful COVID-19 vaccine will make the vaccine affordable and accessible, especially in low- income and emerging markets. Questions remain as to how the vaccine(s) will be priced, to ensure high rates of global immunity and access for the uninsured or under- insured, without being a cost burden to governments, and while allowing innovative researchers to receive a return on their work.
Distribution Priority
Regulatory and advisory agencies have recently released guidance on vaccine dosage distribution, especially in the event that there are not immediately enough doses ready for entire national populations. For example, the U.S. Centers for Disease Control and Prevention (CDC) advised that vulnerable population members like the elderly, the immunocompromised, healthcare workers, and other essential workers would receive the vaccine first. This approach is generally thought to have positive social impacts for communities, workforces, public health outcomes, and economies.

Developing & Emerging Markets
Vaccines in wealthier, developed markets may be taken for granted, but vaccines and immunizations are equally important for developing and emerging markets, for global health and for sustainable global economic development. The eVIN program in India, developed in collaboration with the United Nations Development Programme (UNDP), exemplifies a successful vaccine distribution model for emerging markets. The program has expanded access to essential vaccines to children all over India, through digitization of vaccine stock levels, reduced vaccine waste, and increased training of new personnel in vaccine storage and administration. India has the highest number of annual births of any global country, and eVIN improves access to vaccines to keep our global population healthy.
Life Science Concerns
Social factors also apply to vaccine production, especially where investors’ values-based concerns are involved. Most conventional vaccines are developed with the use of animals, through animal testing of a prepared vaccine candidate, or by growing vaccines in animal products like chicken eggs or mammalian cells. Sometimes, vaccines are grown in human cells. These life science research processes may concern some values-based responsible investors, who apply criteria limiting their portfolio’s exposure to companies who use animals or human samples in their research and development.
Vaccines play a critical role in bolstering immunity and fighting disease in people of all ages. They also save money and lives. The Centers for Disease Control and Prevention (CDC) estimates that among children born between 1994 and 2018 in the U.S., routine childhood vaccinations prevented 419 million illnesses, 26.8 million hospitalizations, and 936,000 early deaths—resulting in $406 billion in direct medical cost savings and $1.9 trillion in total societal cost savings due to childhood vaccination.
— National Infant Immunization week, Centers for Disease Control and Prevention, Vaccine site. April 20, 2020.
CORPORATE GOVERNANCE PERSPECTIVE
Processes & Procedures
Healthcare companies, particularly biomedical and pharmaceutical companies, follow globally accepted procedures for conducting large-scale safe and effective clinical trials for any new medical treatment, device, or drug. However, the urgency and speed to develop a vaccine for COVID-19 puts pressure on the respected processes that normally mean years before a safe and successful vaccine comes to market. Even in this environment of urgency, companies should uphold the established processes and their own governance policies for developing new vaccines with low incidence of adverse events. This applies to companies throughout the whole process – from research and development, to filling and finishing processes, to transportation, to companies doing the actual vaccinating, such as retail pharmacies.
Best Practices
At every stage of the vaccine search, as companies work with public institutions, it’s important for these partners to provide reliable information about the COVID-19 vaccine research process and trials. Companies can use ethical governance principles to reduce misinformation and to play a role in helping distribute a vaccine through various countries’ public health systems.
Public companies are entering new contracts and partnerships with governments or regulatory agencies for the supply and distribution of a COVID-19 vaccine. As with all business dealings, it is important for these companies to uphold strong policies against corruption or bribery with government entities. In particular, corporate partners are striking deals with multiple nations and government agencies; good governance practices reduce conflicts of interest, undue enrichment, unethically secured business, or other untoward commercial behavior. In some industries and countries, bribery scandals have harmed the public and damaged corporate reputations; when creating and enforcing these agreements, companies must exercise best practices in corporate governance, especially related to dealings with government agencies and officials.
Although corporate governance may sometimes be the least headline-grabbing of ESG factors, strong ethical policies and management behavior can help companies steer clear of scandal and enhance the public benefit they aim to provide.
ENVIRONMENTAL EVALUATION
Storage & Energy Resources
As we’ve noted, social factors are arguably the closest- tied ESG factors when discussing vaccines. Nevertheless, there are some environmental impacts behind vaccine research and distribution. Most conventional vaccines must be stored at very cold, even subzero, temperatures. This refrigeration requirement makes vaccine transportation logistically challenging and requires a higher use of energy and resources to keep the vaccine doses in viable condition, traveling at temperatures of between 2°C and 8°C.3
This cold air challenge comes into play for the air cargo sector, which is poised to play a significant role in securely transporting the millions of doses of any new COVID-19 vaccine. The industry faces its own vaccine-related challenges, including: the cold storage requirement throughout transport; the physical security of the vaccine doses; and a dearth of cargo planes. With some modifications, passenger planes could be used to transport the vaccines, but sources estimate that providing the nearly 8 billion vaccine doses to the global population would fill more than 8,000 Boeing 747 planes.4
Sustainability & Packaging
Other environmental challenges play an earlier role in the vaccine production process, including packaging sustainability concerns for vaccine manufacturers. Some countries have laid plans to produce millions of doses of the vaccine candidates that are still in trial, and the doses will be produced before any regulatory approval is granted to the vaccine candidate. At a time when glass vials are scarce,5 the vaccine developers may choose pre-filled syringes as the immunization delivery method. With both dosage methods, manufacturers and distributors have the opportunity to reduce environmental impact by using fewer materials and/or reusing the durable and expensive shipping containers when possible. In 2011, the World Health Organization (WHO) collaborated with Program for Appropriate Technology in Health (PATH) to recommend practices to increase vaccine packaging sustainability, including: “Evaluate potential areas to reduce the environmentalimpact of immunization programs by reducing the export shipping material weight and volume while maintaining the cold chain” and “Request that vaccine manufacturers work with packaging suppliers to increase content of recycled materials.”6 On the cusp of a global, large-scale vaccine release such as the COVID-19 vaccine, it is prudent for these companies to streamline packaging and shipping materials to reduce environmental impacts, to improve the cost of production, and to distribute the vaccine(s) as quickly and efficiently as possible.
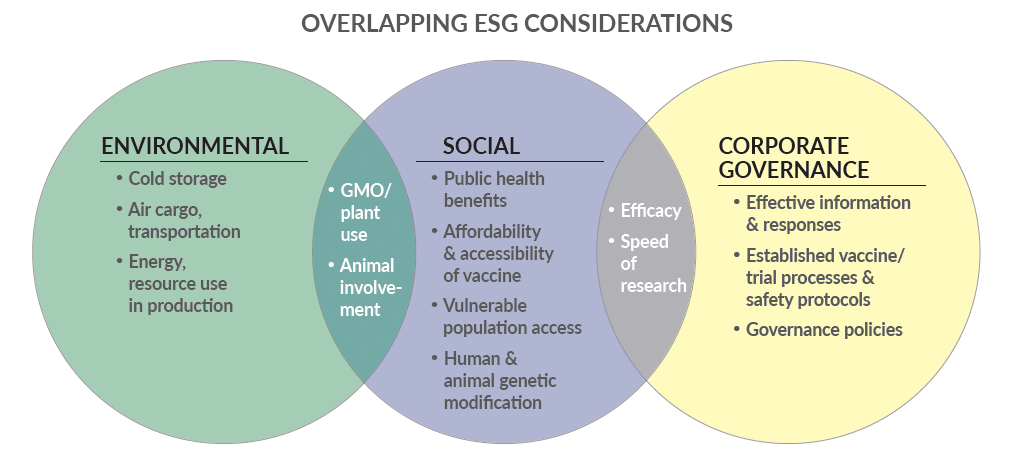
As in the course of traditional investment research, ESG factors are integrated across companies and industries, not existing in a vacuum. Below, and as shown in the graphic above, we present a few overlapping matters pertaining to vaccines.
OVERLAP: SOCIAL & CORPORATE GOVERNANCE
Many health-science organizations and officials emphasize the importance of any new vaccine’s efficacy. Efficacy is tantamount to a vaccine’s success, but many questions related to efficacy have been raised by the COVID-19 vaccine search, including: Will a vaccine actually protect enough members of vulnerable populations? Will it help achieve some level of herd immunity? Is it ethical to distribute a vaccine that is only semi-effective, relatively speaking? Would it provide enough social impact or just force additional vaccine research down the road if the disease mutates too quickly and the semi-effective vaccine becomes ineffective?
Many corporate leadership teams are themselves weighing these questions as they conduct clinical trials or make preparations for distribution of a COVID-19 vaccine. Unfortunately, the answers to some of these questions may only come with time, after a vaccine is developed and rolled out globally.
OVERLAP: ENVIRONMENTAL & SOCIAL
Newer vaccine research, including some of the COVID-19 vaccine candidates, genetically modifies human cells to build antibodies. Some research involves genetically modified organisms (GMO) like plants; certain institutions are researching how to use plants to grow vaccine strains. Someday, receiving a vaccine dose may be as simple as eating a fruit or vegetable that contains the vaccine.7 This delivery method could benefit global markets that do not have robust cold storage systems, or have less-developed healthcare infrastructure, for example. While GMO research may offer some solutions, certain global markets like the E.U. have limiting regulations on GMO use and development, and some responsible investors prefer to avoid GMO investments, based on their individual or institutional values. GMO concerns pertain to both environmental and social factors; some investors are concerned about the long-term public health effects of GMO, or about the impact of introducing GMO seeds or plants to the environment.
Animal testing also overlaps environmental and social factors, given the value some people place on animals’ rights and welfare, and/or championing animals’ protection as part of the natural world. However, animal testing is an integral component of the healthcare sector, as most sector companies are legally required to validate the safety of new and innovative healthcare treatments or medicines through animal trials. In fact, most of the COVID-19 vaccine candidates have been tested on animals to research the vaccines’ safety profiles. Additionally, one company, Anivive Lifesciences is researching the ability to treat COVID-19 with an antiviral medicine developed to treat feline infectious peritonitis (FIP). Because this drug has already undergone extensive animal research, the antiviral can transition to human testing relatively quickly. Some investors values may lead them to avoid investing in companies that utilize animal testing unnecessarily, particularly in instances when the research is not for a direct medical benefit.
A global pandemic is certainly a worldwide challenge that many players – government agencies, corporate entities, private individuals – are working to solve. Namely, the quest for a safe and effective vaccine that stands to impact millions of lives for the better. The search for a COVID-19 vaccine presents unique industry challenges, but also makes an interesting case study for how ESG risks and opportunities can be addressed by companies at the forefront of the vaccine search. Companies and public institutions all aim to develop a safe and effective resolution to the pandemic; ESG factors inform analysis of which entities are mitigating risks and capitalizing on opportunities as they work towards the pandemic’s resolution. Additionally, certain values- based investor concerns are relevant to the vaccine development process. We are all poised to receive positive news about a safe and effective vaccine, and our understanding of companies’ roles in vaccine development continues to be informed by these, and other, environmental, social, and corporate governance factors.
CONCLUSION
At 1919, we have a dedicated team for ESG investing. In this report, we examine how ESG intersects with vaccines, as the pandemic has driven additional investor focus on this important topic. We also highlight the importance of evaluating ESG in investment strategies. We are hopeful of a relatively quick resolution of this public health crisis, and we reiterate our support for vaccines for the numerous positive social impacts they offer, namely public health and safety. When a vaccine is ready, we encourage all readers to consult their medical professionals and to read guidance from local health officials for current and reliable information.

CHRISTOPHER DELPI, CFA
Principal, Equity Research Analyst
Christopher is a Principal at 1919 Investment Counsel, LLC. His primary responsibility is as an Equity Research Analyst covering the Healthcare Sector. Christopher has over 20 years of experience as a Research Analyst with 18 of those years following the Healthcare Sector. Prior to joining the firm, Christopher was a Partner at Philadelphia International Advisors from 2002-2015 where he was The Director of Research and a Research Analyst. Prior to Philadelphia International Advisors, he was a Vice President with The Glenmede Trust Company, following both the Healthcare and Consumer Staples Sectors. Christopher has also held positions at Mercantile Bank & Trust and M&T Bank.
Christopher is a CFA charterholder and received an M.B.A. with a concentration in Finance from Loyola University. He also earned a B.S. in Business Administration with a concentration in Finance, minors in Economics and Psychology, from Towson University/College of Charleston.
Email address: cdelpi@1919ic.com

LEAH FOXX
Social Research Analyst
Leah is a Social Research Analyst for the Socially Responsive Investing Department at 1919 Investment Counsel, LLC in the firm’s Cincinnati, OH office. She has been a member of the firm’s SRI Department since she entered the financial services industry in 2015. Her primary responsibilities include evaluating companies in the Healthcare, Financial Services, and Real Estate sectors, and analyzing relevant social, environmental, and responsible finance topics. Leah publishes comments on company reviews and also writes for the firm’s Social Research Reporter. She
regularly participates in events and meetings related to corporate engagement through memberships in the Interfaith Center on Corporate Responsibility, Principles for Responsible Investment (UN PRI), and the Investors for Opioid Accountability (IOA). Leah is also a member of the SRI Committee at 1919 Investment Counsel.
Leah earned her H.B.A. in Philosophy, Politics, and the Public at Xavier University. She achieved the French professional certificate of conducting business affairs in French, certified by the Chamber of Commerce & Industry of the Paris/Île-de-France region, in 2015.
Email address: lfoxx@1919ic.com
1 Merelli, Annalisa. “Tracking the $5.1B the U.S. spent on COVID-19 Medical Research.” Quartz. July 9, 2020.
2 IQVIA Pipeline Intelligence. IQVIA Institute. December 2019.
3 Harry, Rachelle. “IATA: Air cargo sector must act now to overcome the challenges of vaccine distribution.” Air Cargo News. September 10, 2020.
4 Harry, Rachelle. Air Cargo News. September 10, 2020.
5 Miller, Norman. “The rollout of a COVID-19 vaccine is under threat.” Business Insider. September 21, 2020.
6 Newland, Sophie. Sustainability in Vaccine Packaging. PATH. May 2011.
7 Norero, Daniel. “GMO tomato as edible COVID vaccine? Mexican scientists work to make it a reality.” Cornell Alliance for Science. May 6, 2020.